Necroptosis is a regulated cell death (RCD) similar to apoptosis, the most studied type of RCD. In contrast to apoptosis, plasma membrane degradation occurs in the early stages of necroptotic cells. Because of this, scientists believe that necroptosis causes severe inflammation of the surrounding tissue and plays a role in inflammation-related diseases. However, it is unclear where and when necroptosis occurs under physiological and pathological conditions in vivo.
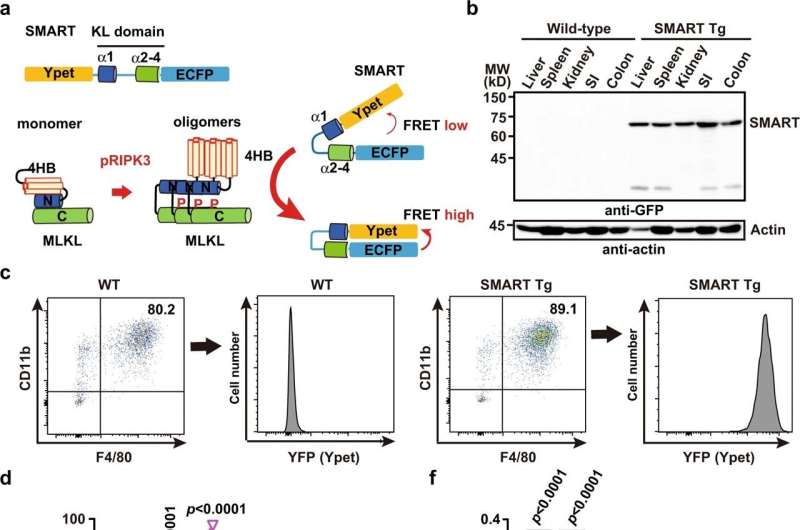
To overcome this problem, Prof.'s group. Nakano previously developed a biosensor for necroptosis called SMART (FRET-based sensor for MLKL activation by RIPK3). This biosensor is based on energy transfer resonance fluorescence (FRET). In a Nature Communications article published in 2018, they managed to characterize necroptosis in vitro using this FRET biosensor.
Now they have gone even further; developed transgenic mice with the SMART FRET biosensor for in vivo monitoring of necroptosis. "The aim of our project was to see when and where necroptosis occurs in vivo and understand its role in the disease context," said Dr. Murai, lead author of the study.
In their experiments, they first confirmed that necroptosis can be controlled in primary macrophages derived from SMART-Tg mice or in embryonic fibroblast clumps. They then implemented a cisplatin-induced acute kidney injury model in SMART-Tg mice.
“After much trial and error, we were finally able to monitor the execution of necroptosis in proximal tubular epithelial cells in SMART-Tg mice injected with cisplatin. Two-photon microscopy is essential for the effective monitoring of cell death in vivo," said Prof Nakano, lead author of the study.
"We believe that SMART Tg mice are a promising tool for visualizing necroptosis in vivo and helping us better understand the role of necroptosis, a relatively new form of cell death, in disease pathophysiology."
The findings are published in the journal Communications Biology .
Further information: Shin Murai et al., Generation of transgenic mice expressing the FRET biosensor, SMART, that responds to necroptosis, Communications Biology (2022). DOI: 10.1038/s42003-022-04300-0
Provided by Toho University
Excerpt : FRET-based biosensor visualizes the performance of necroptosis in vivo (9 December 2022) Accessed 17 December 2022 https://phys.org/news/2022-12-fret-based-biosensor-visualizes-necroptosis-alive.html- from
This document is protected by copyright. Except for fair trade for personal study or research purposes, no part may be reproduced without written permission. Content is provided for informational purposes only.


.png)
Post a Comment
Post a Comment